Compass Listeria (RTU)
ISO 16140-2 validated alternative method for the detection of Listeria monocytogenes and Listeria spp. COMPASS® Listeria is a rapid alternative method used for the detection of Listeria monocytogenes and of Listeria spp. in food products, and in environmental samples. It features a single step of selective enrichment in Fraser ½ broth, followed by subculture onto COMPASS ® Listeria Agar. Enrichment can be performed at 37°C for 18 to 24 hours, or at 30°C for 22 to 28 hours.
This method is certified NF VALIDATION, according to the validation protocol NF EN ISO 16140-2 of 2016 for all human food products and samples from the industrial production environment. The reference method used for the validation is the standard NF EN ISO 11290-1 of 2017.
Available in 20 and 120 plate formats Ø 90 mm.
This medium is also available in dehydrated format of 500g (BK192HA) in addition to the corresponding selective and enrichment supplements. To be used with freeze-dried selective supplement (BS07108) and Enrichment supplement (BS07008).
In compliance with regulatory requirements, a USDA permit (VS 16-3) is mandatory for shipments from Canada to the USA due to the inclusion of peptone, an animal byproduct.
Refer to the certificate available on the NF VALIDATION website for the validity end date of the method. In the context of the label NF VALIDATION, sampling sizes greater than 25 g were not tested. ISO 16140-2 validated alternative method for the enumeration of Listeria monocytogenes. The COMPASS® Listeria method is also used as a rapid alternative method for the enumeration of L. monocytogenes in human food products and environmental samples, by surface or deep plating.
Refer to the certificate available on the NF VALIDATION website for the validity end date of the method.
Normalized method for the detection and enumeration of Listeria monocytogenes et Listeria spp.
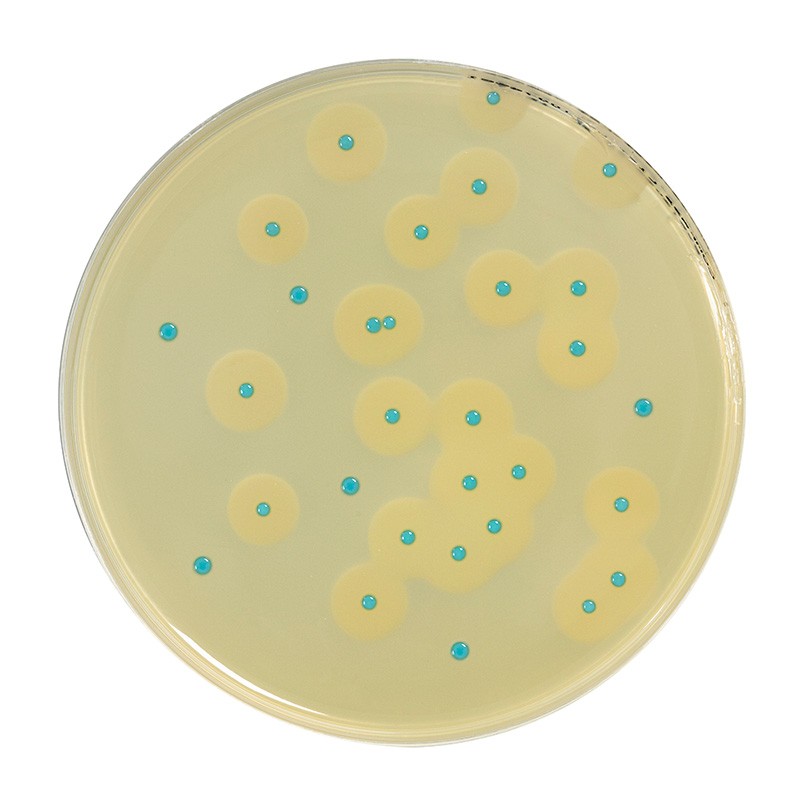
The formulation of the COMPASS® Listeria Agar corresponds to that recommended in the international standards NF EN ISO 11290-1 and NF EN ISO 11290-2. (Refer to COMPASS® Listeria (ISO11290-1 & -2) data sheet.) COMPASS® Listeria Agar is the first mandatory isolation medium in the L. monocytogenes and Listeria spp. detection protocol, and the only medium in the L. monocytogenes and Listeria spp. enumeration protocol.
The peptones and growth factors (yeast extract, sodium pyruvate and magnesium sulfate) favor the growth of Listeria monocytogenes. Listeria hydrolyze the 5-bromo-4-chloro-3-indolyl-β-D-glucopyranoside (or X-β-glucoside). The resulting product is subjected to an oxidative dimerization that forms a blue precipitate on the colonies.
Phosphatidyl-inositol is used as a substrate for the detection of phospholipase C of Listeria monocytogenes. When it is degraded, an opaque precipitate is formed around the colonies. Secondary microflora is inhibited by the association of lithium chloride and a judicious mixture of selective agents that include several antibiotics and an antifungal agent.
Related Products